NASA made a monumental announcement on September 28: the discovery of liquid water flowing on the surface of Mars, the Red Planet
Before everyone gets excited, there are two caveats to this discovery. The first is primarily that it isn’t pure water, but a saline solution containing various salts. The second is that the discovery was only made under certain atmospheric temperatures, indicating that the flow is seasonal.
Ok, you can get excited again now because this is still a monumental discovery for the “follow the water” mantra.
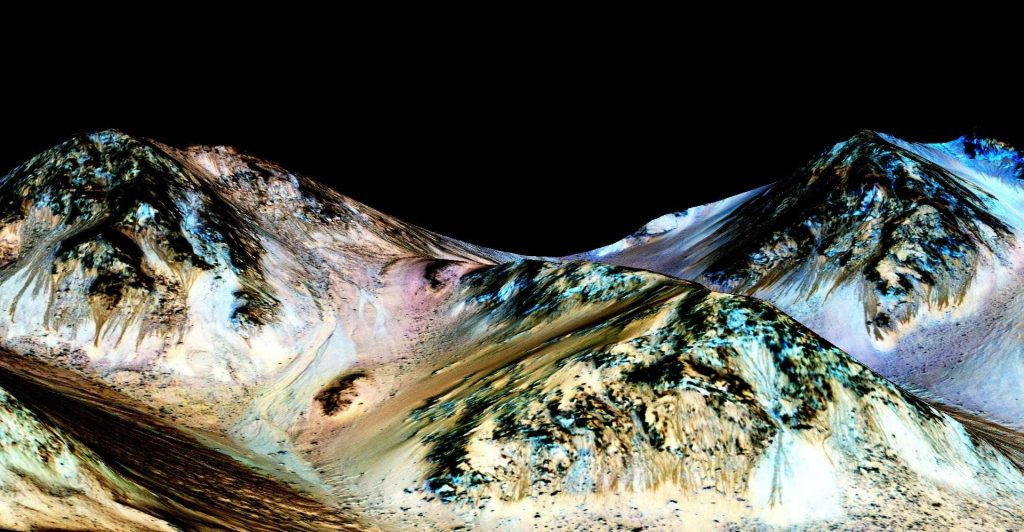
The discovery started with the launch of the Mars Reconnaissance Orbiter (MRO), a satellite equipped with imaging spectrometry that began monitoring Mars in 2006. First observed in 2010, the MRO recorded images of flowing hydrated salts composed on dark streaks, moving down large peaks during warm seasons and stopping during the colder seasons. These downhill flows, known as recurring slope lineae, were hypothesized to contain liquid water, Lujendra Ojha of the Georgia Institute of Technology and lead author of the report on these findings said in a NASA press release.
One of the complications for finding liquid water on Mars is its very low pressure atmosphere, comparatively more than 100 times smaller than that of Earth’s. At the pressure of 600 pascals, liquid water can’t exist as a pure solution because it is below water’s triple point This means that you can only find water in two forms: as a gaseous vapor at higher temperatures within the atmosphere and as the solid ice that you’d find underground in cooler regions. The way around this, as NASA has recently observed and hypothesized for years, is for the water to be highly salinated.
At temperatures above -23 degrees Celsius, NASA discovered that the hydrated minerals likely contained chlorates and perchlorates; proposed to be magnesium perchlorate, magnesium chlorate and sodium perchlorate by interpreting the wavelengths of their imaging equipment. For those who took and dreaded Gen Chem, you’ll remember that when you dissolve a substance into a solvent such as water, it lowers the freezing point. The more concentrated the mixture becomes, the lower the freezing point extends. The salts that were present are known to draw in water from the atmosphere through a process known as deliquescence and are also known to prevent water from freezing far below its natural freezing point, according to tests on Earth.
So, let’s get to the point: What does this have to do with aliens?
Well, deliquescence isn’t a process that’s unheard of, and it happens right here on Earth. In South America, the Atacama Desert’s Yungay region has been the target of interest in finding an environment that simulates that of Mars. One of the signature salts in the Atacama are perchlorates capable of absorbing water from the atmosphere. Only within the past decade was it discovered that even in these conditions, endolithic cyanobacteria (living within porous rocks or grains) formed colonies dependant upon the absorbed water to carry out metabolic processes.
So while signs of intelligent life are absent, the chances that there may be extra-terrestrial bacteria are a bit more possible than they were two weeks ago.
While no definitive answers exist on where this flowing Mars water comes from, there are a few hypothesis other than deliquescence. One such hypothesis is that this water may come from frozen underground reserves that heat up and liquify in the warm season, bringing water to the surface. The other is that gritty rocks on the surface retain saline solution, which escapes during the warm season, but this theory still leaves some questions unanswered. Hopefully, with funding, NASA can one day hope to answer the important questions with direct analysis of the the RSL samples and find if there really is life of any sort outside of Earth.



Comments are closed.